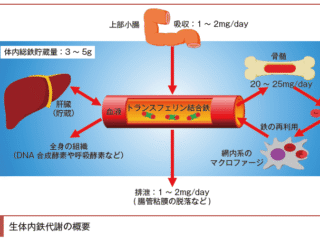
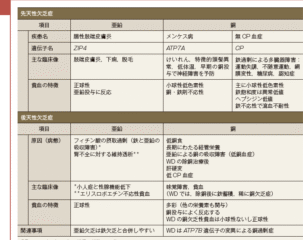
文献
- 小船雅義.鉄剤の臨床効果と使用上の注意.p44-49. 日本鉄バイオサイエンス学会治療指針作成委員会(編).鉄剤の適正使用による貧血治療指針改訂[第 3 版],札幌 , 響文社,
- Komatsu N, Arita K, Mitsui H, et al. Efficacy and safety of ferric citrate hydrate compared with sodium ferrous citrate in Japanese patients with iron deficiency anemia: a randomized, double-blind, phase 3 non-inferiority study. Int J 2021; 114: 8-17.
- 小松則夫 , 秋澤忠男 , 有田好城 , ほか.鉄欠乏性貧血患者を対象とした ferric citrate hydrate の 鉄 補 充 効 果. 臨 床 血 液 2021; 62: 1583-1592.
- Ikuta K, Shimura A, Terauchi M, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of intravenous ferric carboxymaltose: a dose-escalation study in Japanese volunteers with iron-deficiency Int J Hematol. 2018; 107: 519-527.
- Ikuta K, Hanashi H, Hirai K, et al. Comparison of efficacy and safety between intravenous ferric carboxymaltose and saccharated ferric oxide in Japanese patients with iron- deficiency anemia due to hypermenorrhea: a multi-center, randomized, open-label noninferiority study. Int J Hematol. 2019; 109: 41-49.
- Ikuta K, Ito H, Takahashi K, et al. Safety and efficacy of intravenous ferric carboxymaltose in Japanese patients with iron-deficiency anemia caused by digestive diseases: an open-label, single-arm Int J Hematol. 2019; 109: 50-58.
- Kawabata H, Tamura T, Tamai S, et al; Study Group. Correction to: Intravenous ferric derisomaltose versus saccharated ferric oxide for iron deficiency anemia associated with menorrhagia: a randomized, open-label,active-controlled, noninferiority study. Int J Hematol. 2022; 116: 976-977.
- Kawabata H, Tamura T, Tamai S, et al; Study Group. Intravenous ferric derisomaltose versus saccharated ferric oxide for iron deficiency anemia associated with menorrhagia: a randomized, open-label, active-controlled, noninferiority study. Int J 2022; 116: 647-658.
- Kawabata H, Tamura T, Tamai S, et al; Study Group. Intravenous ferric derisomaltose for iron-deficiency anemia associated with gastrointestinal diseases: a single-arm, randomized, uncontrolled, open-label Int J Hematol. 2022; 116: 846-855.
- Sugimura M, Ohtani Y, Tamai S, et al. Ferric derisomaltose for the treatment of iron deficiency anemia with postpartum hemorrhage: Results of a single-arm, open-label, phase 3 study in Japan. J Obstet Gynaecol Res. 2023; 49: 946-955.
- Kalra PR, Cleland JGF, Petrie MC, et al; IRONMAN Study Group. Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): an investigator-initiated, prospective, randomised, open-label, blinded-endpoint trial. Lancet. 2022; 400: 2199-2209.
- Ponikowski P, Kirwan BA, Anker SD, et al; AFFIRM-AHF investigators. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double- blind, randomised, controlled Lancet. 2020; 396: 1895-1904.
- 中尾喜久 , 衣笠惠士 , 前川正 , ほか . 鉄欠乏性貧血の治療 , とくに非経口鉄剤の応用について . 日本臨床1956; 14: 843-852.
- 内田立身 , 河内康憲 , 渡辺礼香 , ほか . 鉄欠乏性貧血の静注療法における鉄投与量の再検討 . 臨床血液1996; 37: 123-128.
次のページへ
page:45
「鉄欠乏性貧血の診療指針」の記事に関する転載、商用利用についてはこちらのリンクをご確認ください